What Hold's The Atomic Nucleus Together?
The Atomic Nucleus
As the atomic model was developed by scientists, what was not clear was how the protons stayed together tightly packed into such a small volume, as defined by the small charge radius of the nucleus. Because of the strong repulsion between like electrical charges, the Coulomb force at such short distances is enormous and should explode the nucleus with great force. However, with the exception of some “radioactive” atoms the nuclei always remain stable.
What holds the atomic nucleus together?
This question has been at the heart of nuclear science since 1932 after James Chadwick’s discovery of the neutron, which showed that the nucleus is made from protons and neutrons.
The argument goes like this. There is a strong electric repulsive force between the positive proton charges, so how does a nucleus consisting of many protons and neutrons stay bound together in such a small volume? Obviously, there must be another force that counteracts the electric repulsion between protons.
The concept of a strong attractive nuclear force between protons and neutrons was quantitatively described in 1934 by Hideki Yukawa by introducing the meson, a “virtual” particle. A virtual particle cannot be directly detected and occurs over very short intervals of time and space with correspondingly indefinite energy and momenta.
From this beginning more than eighty years ago, in current theory, the nuclear force exists as a “residual” player in the “strong interaction” that holds the quarks of a “hadron” together.
This nuclear force of current science is strongly attractive, and unlike electricity and magnetism, decreases faster than the inverse square of distance limiting it to a very short range, in the magnitude of around one femtometer (10-15) between centers of the protons and neutrons. At distances less than 0.7 fm between centers, the nuclear force is considered to become strongly repulsive.
The strong force is designated as one of the four fundamental forces of nature. Without a strong force it is believed the nucleus cannot be held together. The physical reality of the strong force is not an open question by current science.
The Neu Theory Model
The Neu Theory model of nuclear structure does not require the use of a strong force as it claims that the nucleus is stable because:
There is no electric repulsion inside the nucleus because there is no electric charge inside the nucleus!
The key word is inside. In Neu Theory, all the individual proton electric charge shell particles have migrated above and outside the surface of the neucleonic cellular membrane in concentric “Z” layers of isotropic spin energy tension bands. These tension bands act together as a compressive binding force that holds the neucleons inside a nuclide bound together. To repeat, the electric charge shell particles are never inside the body of a matter object, they are always outside the body of the neutral matter surface.
The Neu Theory neucleon (note different spelling), is a single cell nuclear object with one, two, or three cores below an electric charge shield. Specifically a neutron cell (n), a deuteron cell (H2+), or a helion cell (He3++). See Diagram The Three Neucleons.
What is significant to the model, is that there are no free proton cores in a nuclide, they are all captive to a neutron cell as deuterons or helions, with the proton’s charge shell having migrated above the neutral S3 surface of the neucleon cell, or above the composite S3 neutral membrane surface of a cluster of neucleon cells.
The Neu Theory nuclear model works, because positive electric charge is hypothesized as an elementary particle, a thin discrete shell of isotropic spin energy moving at the accelerating speed of light, that can be topologically “detached” from the proton’s S1 spinning matter surface, to “migrate” above the neutral S3 non-spinning surface of the neucleon membrane. When the spinning captive proton cores are immersed in the neucleonic plasm matter, they are no longer directly “jacketed” with their charge shells. See Diagram The Three Neucleons.
In principle, there can be no charge below the neutral neucleonic membrane. This is very different than the current view, that electric charge is an intrinsic property that is permanently contained within the bodies of the quarks (or electrons) and can never leave.
With free proton and electron ions, the electric charge shells are directly touching a spinning Type I matter surface (S1 for protons, and S2 for electrons) See Diagram The Electric Charge Shells. With neucleons, the electric charge shells are directly touching the S3 neucleonic membrane surface(s), which is non-spinning Type I matter. All spinning cores are immersed within the individual non-spinning compressible Type II matter plasm particles contained within a deformable non-spinning neucleonic membrane. See Diagram The Three Neucleons.
To understand nuclear structure we need to clearly understand the form and properties of matter and electric charge.
The early 1960 model of the proton is:
- a sphere with a small definite volume
- a fixed mass called its rest mass
- a fixed positive electric charge
- a fixed spin
- a fixed magnetic dipole aligned with spin.
This is all true and verifiable.
The size of a free proton or some nuclide is defined by its charge radius. The charge radius is a well measured nuclear quantity, where even a small size difference between the nuclei of different isotopes can be determined. For comparative purposes the charge radius can be used to define the physical volume of a nuclide. The Neu Mass & Charge Radii Table uses these radii to calculate the volume and other values of nuclei based on this measured size. If the charge radius is changed the calculated values will adjust.
The Distribution of Charge
A key question must still be answered. How is the positive electric charge distributed with a proton or nuclide? Where does charge reside?
This is where the Neu Theory model fundamentally departs from current science.
Current Science: The historical view of charge distribution was that the total electric charge is uniformly distributed throughout the volume of the proton. The current view by the Standard Model of particle physics is that the proton’s positive charge is actually a composite quantity made from three fractional charges (+2/3, +2/3,-1/3) carried by the three quarks that constitute the proton. This did not change the historical view that electric charge is contained within the body of the proton.
Neu Theory: the model hypothesizes that total electric charge of the free proton (and the electron) is uniformly distributed on a neutral spinning Type I matter surface; while the total electric charge of a nuclide is uniformly distributed on a neutral non-spinning Type I matter surface(s), as a discrete thin layer or multiple layers (each layer has a fixed volume) of isotropic spin energy moving at the accelerating speed of light.
In principle there is no charge allowed within the nuclide body. This is because there cannot be a de-linked energy form (spin or rise) within the matter body of a neutron, as that would make the neutron topologically greater than one unit of number, which is not allowed by the rules of number. As the nuclide is made from neucleons, which are just neutrons with one, two, or three cores, it follows there will not be any charge below a nuclide.
With a specific nuclide the multiple electric charges are uniformly distributed in thin layers of isotropic spin movement/energy that act as compression bands that are discrete from the non-rotating surface(s) of the neutral neucleonic membrane matter structure. Electric tension within the naturally accelerating spin movement/energy bands is what keeps the neutral single cell neucleon molecules bound together in a cluster.
There is no electric charge within any nuclide no matter how many protons it contains!
The postulating of a “strong” fundamental attractive force of nature that overrides the immense force of Coulomb repulsion is not required. The electric force is sufficient for nature to do its magic.
The Two Charge Shells
The Neu Theory electric charges are elementary particles, modeled as two equal topologically mirror-split forms of de-linked spin movement/energy (elementary forms [6+] & [6-]) with their own measurable elementary physical properties (i.e., shape, size, quantity and structure). These properties are discrete from the physical properties of the spinning neutral matter of the proton and the electron they surround, and discrete from other charge shells in a multiple charge layer nuclide.
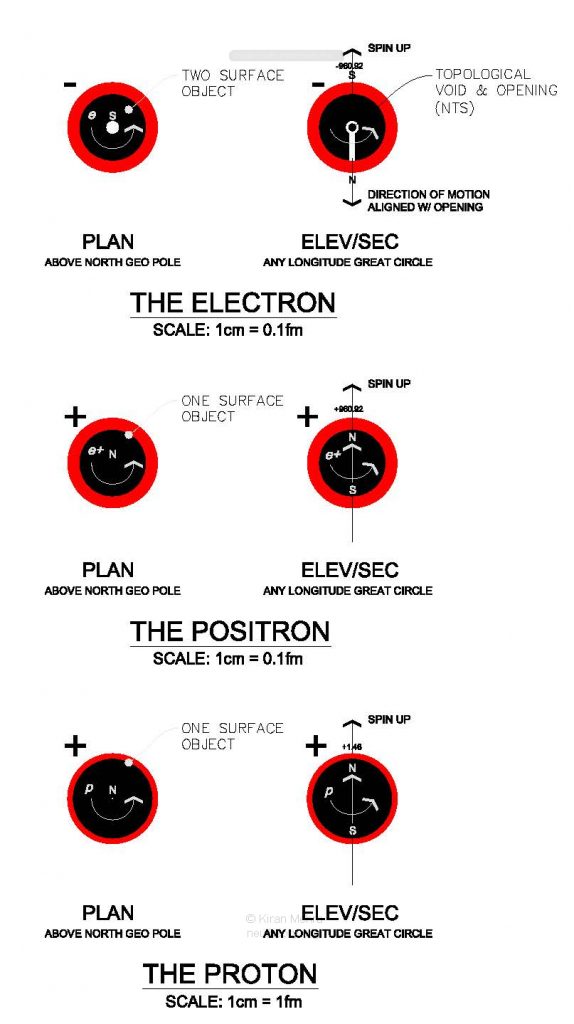 Figure 4.2 – The Electric Charge Shells
Figure 4.2 – The Electric Charge Shells
The positive and negative electric charge shells are exactly alike except they are topologically mirrored. As the same specific volume of charge surrounds different size balls their charge shell thickness is proportionately different. The electron charge shell is approximately 125 times thicker than the proton’s charge shell.
As the initial working assumption, Neu Theory considers the physical volume of charge energy to be similar to the physical volume of Type I matter. One neu of Type I spinrise matter has an absolute volume of ∼2.502 x 10-45 m3. This means that 0.000833 neu of electric charge spin energy has an absolute volume of ∼2.084 x 10-48 m3, with each charge shell having exactly one half of that volume at ∼1.042 x 10-48 m3.
Fusion
Fusion is the merging of the charge shields, that surround two or more nuclides, when the nuclides are forced to touch each other, thereby forming a single charge shield surrounding a larger number nuclide. The total number of individual charge shells before and after remains the same.
In Neu Theory, protons by themselves cannot fuse together to make helium. A deuteron has to be made first. A proton can then fuse with a deuteron making helium-3, and deuterons can fuse with other deuterons making helium-4 and larger nuclides. See Diagram The Alpha – He4.
The merging of two galaxy electric supercells is also fusion albeit at a much larger cosmic scale.
Fission
Fission is the separation of a larger number charge shield into two or more smaller number charge shields. This occurs when a large deuteron number nuclide can no longer maintain its stability and breaks apart into one or more smaller deuteron number nuclides that carry their charge shell layers with them. The emission of a two deuteron alpha particle with its two charge shells is a common example of fission. The total number of individual charge shells before and after remain constant.
Internal Nuclear Forces
(See Figure 4.3 – Section @ Nuclide Surface). This is a schematic drawing of a cross section at the neucleonic membrane surface of any neucleon cell. This shows the relationship between the three matter particles (core, membrane and plasm), shown in black and grey, the positive electric charge shield, shown in red, and physical space shown in light blue.
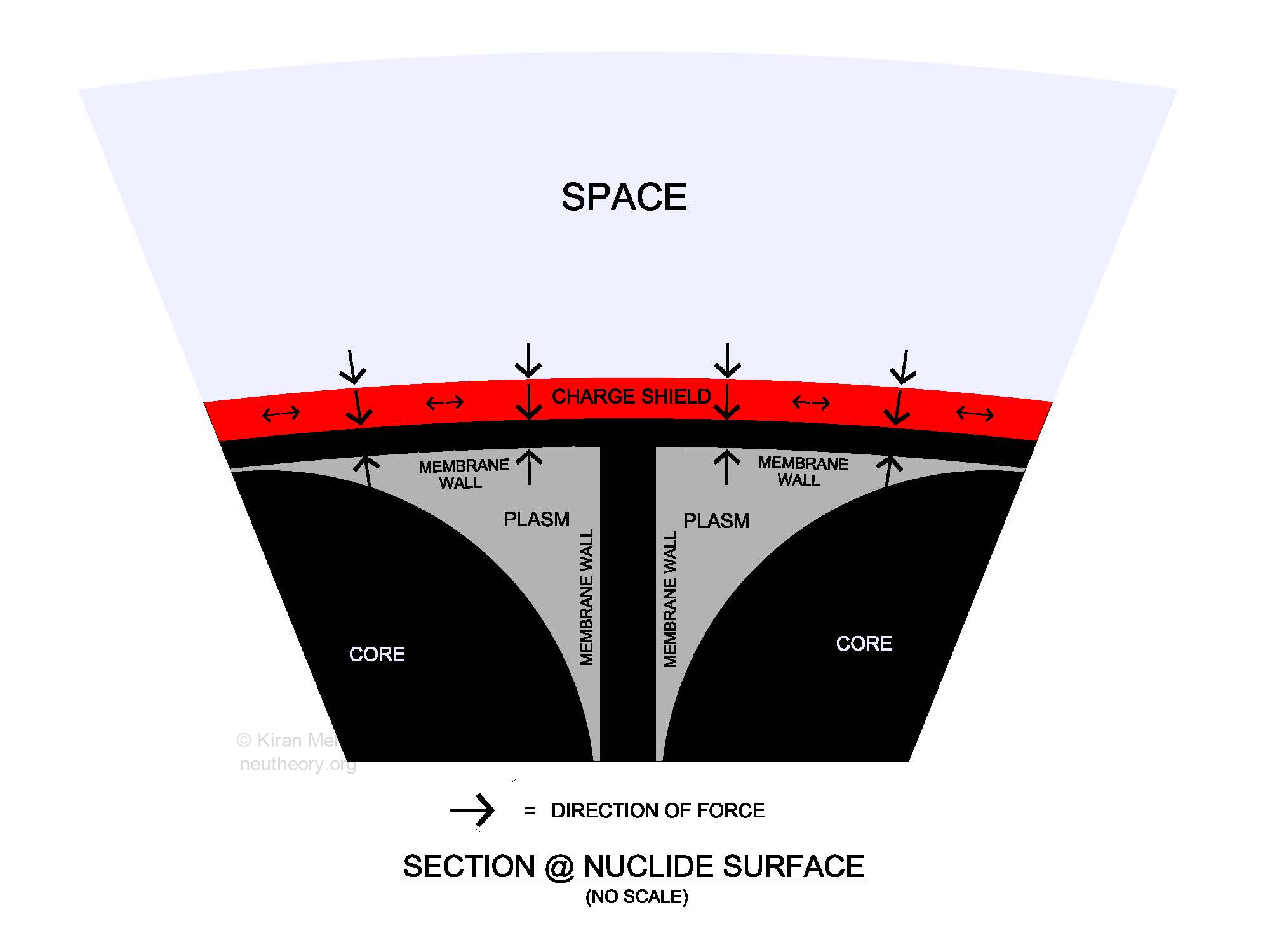 Figure 4.3 – Section @ Nuclide Surface
Figure 4.3 – Section @ Nuclide SurfaceAll five entities are topologically discrete with a definable boundary between each other. The colors never mix. It should be noted that there is no space below the electric charge shield. The net nuclide magnetic field, usually indicated with a light green shade, is not shown in this diagram.
Internal nuclear forces in the Neu Theory Model are the ordinary everyday g-forces of nature, nothing special is required. Three of the four natural accelerations play the major role:
- G-spin/magnetic alignment of the spinning cores [4] keeps the individual neucleon cores aligned with a common nuclide spin/magnetic axis. The plasms, membranes and the nuclide body as a whole do not spin.
- G-rise pressure of the neucleons [5]. The outer S3 surface of each neucleon cell is in direct physical contact with the outer S3 surface of an adjacent neucleon cell, or the inner surface of the common electric charge shield. The g-rise of each neucleon adds to create a g-rise of the nuclide as a body.
- G-fall compression of the electric charge shells [6+]. The positive charge shield is in direct contact with the neutral neucleonic S3 surfaces. The g-fall tension within the isotropic spin movement/energy shell becomes equal to nuclide g-rise, holding the neucleon cluster physically bound together with a specific volume. The natural pressure of g-fall is directly opposite to the pressure of g-rise, towards a minimum sphere packing geometry. Nuclide average densities vary from 12% (H2), to almost 74% (Pb208) of absolute density.
The g-rise pressure from physically accelerating matter and the g-fall compression from physically accelerating isotropic spin movement/energy find a dynamic balance that creates a well defined nuclide size. See Neu Mass & Charge Radii Table – Columns AA and AE.
The small g-rise pressure from space [8] is external to the charge shield, and directly opposite the pressure from the g-rise of matter and in the same direction as the g-fall of charge. Space pressure does not significantly impact internal nuclide structure.
The positive electric force field [7+] of the nuclide, the negative electric force fields [7-] of the orbital electrons, and the residual potential energy of the electric hollows [10] do not impact internal nuclide structure. The nuclide structure remains the same for a neutral or fully ionized atom.
The spinfield [5] form of the nuclide extends into space beyond the atomic radius, and does not impact internal nuclide structure.
Historical Commentary on Nuclear Structure
It is the writer’s opinion that historically, since 1932, the internal structure of the atom, repository to all of nature’s ordinary matter, has not been clearly understood. This lack of clarity at the subatomic scale began after the discovery of the neutron in 1932 and has continued to date. There are two main reasons for this:
- The first reason, is in the definition of matter and mass, and its relationship to energy. Using Einstein’s E=MC2 equation as the justification, energy is not only considered as equivalent to mass, but is also considered as identical to mass in having the property of inertia. This has allowed a theoretical model of the proton and neutron which (somewhat similar to the atom) is modeled as nearly empty space, with most of the well defined rest (inertial) mass provided by force carrying energy particles called “gluons” which add to the smaller inertial mass from the elementary matter carried by the three point-like fractionally charged quarks.
In Neu Theory, homogenous inertial mass is one of the elementary physical properties of matter (linked spin and rise energy) contained in the cores [1a][1b], membranes [2a][2b] and plasm [3], three of the five “elementary fermions” described by the model. These three primal objects are the only matter objects in the universe, and the only source of mass in nature. Mass in this model is defined as the ratio between the bound rise movement/energy content of a matter body, and the free rise energy inertial field provided by space [8]. The positive and negative electric charge shells [6+][6-], the other two elementary fermions, carry a fixed quantity of spin movement/energy, but not inertial mass. The energy of motion (momentum) [9], and the energy of radiation (photons) [11], has a mass equivalence but does not add inertial mass. - The second reason is that the physical identity of electric charge has not been clearly established. This has allowed a model of the proton, by current science, where its well defined positive charge (e+) is constructed from three discrete fractionally charged quarks (+2/3, +2/3, -1/3) physically contained within the body of the proton that add up to a plus one charge (+1). The neutron with no evidence of any electric charge, is also considered to be made of three fractionally charged quarks (+2/3, -1/3, -1/3) that cancel each other adding up to zero charge. However, the equal and opposite charge (e-) of the electron, the other half of electric energy, is not given such a fractional structure.
Basic Neu Theory Nuclear Concepts
The following concepts are used by Neu Theory in its description of atomic nuclear structure and behavior.
Note: These basic nuclear concepts are specific to Neu Theory and are not used by current science:
- A nuclide is a positive electric charge shielded cluster of single cell neucleons.
- A neucleon is a neutron cell with 1, 2 or 3 cores below a positive electric charge shield. See Diagram The Three Neucleons. The charge shield is made from the fermionic addition, by topological layering, of the positive electric charge shells of captive proton cores. The positive electric charge shells that were once directly on the spinning matter surface of protons, have now migrated above the non-spinning matter surface of the neucleonic membrane. All nuclides (except H12, H2 and He3) are made up of two or more neucleons.
- There is no electric charge (topologically split absolute spin movement/energy), below the neutral nuclide membrane surface. All of the volume of electric charge energy, and the electric force field, exists above the neutral nuclide membrane Type I matter surface.
- The direction of the g-fall force by the natural acceleration of positive electric charge is exactly opposite the direction of nuclide neutral matter g-rise pressure, providing a dynamic balance. See Diagram Section @ Nuclide Surface.
- There can be no space (de-linked free-rise movement/energy), below the electric charge shield surface, and below the nuclide membrane surface. All of nature’s space exists above, and is discrete from, the neutral nuclide membrane surface, and the electric charge volume.
- All neucleon cells, of any nuclide, have Type I matter surface membranes that are equal in mass, volume, and density. The thickness of the neucleon membranes in a nuclide will vary, depending on, if the membrane encloses a deuteron or a neutron cell. The membranes always remain discrete, and touch each without any space in between adjacent surfaces. Individual neucleon membranes, adjust themselves as needed, to fit within the total spheroid nuclide volume by adjusting their shape and thickness.
- All neucleon cells (both deuterons and neutrons), of a specific nuclide, have 0.000833u mass Type II matter plasms that are equal in volume and density. All the individual plasms of a nuclide, homogeneously adjust in volume and density as needed, until an equilibrium size of the nuclide is maintained.
- All neutron cores in a nuclide are equal, with an invariant 0.998623u mass, volume and density.
- All captive proton cores in each specific nuclide are equal at absolute density, however the core mass and volume will vary with nuclides. See Neu Mass & Charge Radii Table.
- All mass reduction (or gain) of a nuclide, comes from the captive proton cores only, which are equally reduced (or increased) in mass and volume. Neutron core mass is never reduced.
- All neucleon cores are initially assumed to be perfectly spherical. It is unknown if a core can deform from a spherical shape under pressure.
- All changes in size of a nuclide, as measured by its charge radius, come from a corresponding change in the volume and density, of 0.000833u Type II plasm matter, and the change in mass and volume (but not density) of captive proton cores.
- All cores are perpetually spinning in the same universal direction. In this model, the time for one revolution is called quantum spin (qs). The quantum spin of the neutron cores (and free protons) is invariant at 1.764 381 x 10-23 seconds, which is equal to a frequency of 5.667 711 x 1022 revolutions per second. The quantum spin of captive proton cores changes with each nuclide and is based on mass and size alone as density remains constant. See Neu Mass & Charge Radii Table – Column AX. It is hypothesized, that the spin of each core is perpetually synchronized with like (same mass) nuclide cores throughout the cosmos. The spins never stop accelerating, and the right hand direction of core spin never changes.
- In a stable nuclide, the spinning cores within a neucleon never touch each other or the inner S2 surface of the enclosing neucleon membrane. There is always a “thin” film of neucleon plasm matter between the spinning core(s) and the non-spinning membrane. There is assumed to be pressure, but no “friction” or “spin transfer” between the dense spinning core, and the homogenous less dense non-spinning plasm.
- In nuclides, all spinning cores are aligned parallel or anti-parallel, with one common nuclide spin/magnetic axis. The core spins can only be in one of two directions to this common axis. Cores with spins in the same direction resist interaction. Cores with spins in opposite directions are neutral to interaction.
- An unbalanced net internal spin, does not cause the charge shielded nuclide to rotate as a whole. All core spins are contained within the non-spinning neucleon plasm.
- All spinning cores are magnets with a fixed polarity aligned with the spin axis, and a fixed field strength. Neutron cores and captive proton cores have an opposite magnetic polarity. In all neucleons, and in any nuclide, the neutron core magnetic field strength is hypothesized to remain constant. The captive proton magnetic field strength, is hypothesized to increase as its mass and size are reduced.
General Nuclear Terms
The following terms are used by Neu Theory in its description of atomic nuclear structure and behavior. Most of the nuclear terms are also used by current science, however, this is the meaning as used in this model. A few terms are specific to the Neu Theory model:
- alpha emission – The radioactive emission of a He-4 nuclide by fission.
- atomic number – The atomic number (Z), is equal to the total number of captive proton charge shells.
- beta decay – The “spin-off” and inversion by the neutron membrane, from a free neutron cell, or a neucleon cell that is part of a nuclide. The “little bang” of Neu Theory. (See Figure 3-1 – The Little Bang Transformation).
- captive proton – A proton that is held within the neucleonic plasm of a neutron cell. The proton is below the S2 surface of the neucleonic membrane, with its charge shell above the S3 surface of the neutron cell. There is no charge below the S3 surface. (See Figure 4.4 – The Three Neucleons).
- charge shell – A layer of free isotropic split spin movement/energy closing at the accelerating speed of light surrounding the spinning S1 neutral surface of the proton and the spinning S2 neutral surface of the electron.
- charge shield – One or more electric charge shells, surrounding the S3 membrane surface(s) of a neucleon or a neucleon cluster.
- cluster number – The number of neucleon cells in a neucleon cluster (C).
- deuteron number – The number of deuteron (D) neucleon cells in a neucleon cluster. This is equal (with the exception of a few radioactive atoms) to the atomic number (Z). D equals Z.
- deuteron synthesis – The “spin-on” inversion of an electron membrane over 2 proton cores, creating a neutron cell with a captive proton. The transformation from the b-state into the ab-state. See Figure 4.3.
- electric quadrupole moment – Neu Theory considers the electric quadrupole moment value an indication of the shape of a nuclide around its spin/magnetic axis. A zero (“0”) value considers the nuclide as a sphere. A positive (+) value indicates a prolate spheroid (cigar) shape. A negative (-) value indicates an oblate spheroid (disk) shape. See Neu Mass & Charge Radii Table – Column AB.
- electron – The electron (e-) shell is the free detached neutron S2-S3 surfaces of the neutron cell, and a quantum part of the b-state of matter. After “spin-off”, the membrane flips inside-out, and curls up into a small spinning magnetic ball atabsolute density, and after the “little bang” becomes “jacketed” with the negative electric charge shell [6-]. The neutron cell’s S3 surface never disappears. A primal surface can never be removed from nature. The S3 surface becomes a topological void at the electron’s center, ready to reemerge as a S3 neucleonic membrane with the “spin on” process.
- electron capture – The “spin-on” by an electron membrane, over a helion proton in a nuclide, making a neutron and a deuteron. See (Figure 4.7 – The Triton – *H3).
- fission – The splitting of a large number neucleon cluster into 2 (or more) smaller clusters.
- fusion – The union of separate neucleons into a single larger number neucleon cluster, by the merging and layering of the individual neucleon electric charge shells into one nuclide charge shield.
- gamma ray absorption – The absorption of a gamma ray photon by a nuclide.
- gamma ray emission – The emission of a gamma ray photon by a nuclide.
- helion – A neutron cell with two captive protons and two captive charge shells. The helion is the nucleus of the Helium-3 atom. See (Figure 4.6 – The Helion – He3). The helion is stable as a free neucleon, but is unstable when part of a multi-neucleon atom. For example; the helion in radioactive *Carbon-11, becomes a deuteron and a neutron, as part of a stable Boron-11 atom, by the emission of a positron with a half life of 20 minutes. See (Figure 4.16 – Positron Emission).
- helion number – The number of unstable helion neucleons in a neucleon cluster. Each helion contributes 2 captive charge shells. For example; the radioactive *Nitrogen-12 atom, is hypothesized to have a helion number of 2. Both of the two helions become deuterons as part of stable Carbon-12, by the emission of a positron with a half life of 11×10-3 seconds.
- helion synthesis – The capture of a proton by a deuteron in a stellar environment. An alternate method is the beta decay of the hydrogen-3 nuclide. See (Figure 4.6 – The Helion – He3).
- isomeric transition – The emission of a gamma ray from an atom when the nucleus is in a metastable state.
- mass number – The mass number (A), is equal to the total number of spinning cores in a nuclide.
- metastablity – An extended lifetime of a higher energy nuclear state (nuclear isomer), before spontaneous changing back into the lowest energy state, with the emission or emissions of gamma rays. For example; Stable Molybdenum-98 atoms are bombarded with neutrons making radioactive * Molybdenum-99 with a half-life 66 hours, which then decays into *Technetium-99m with a half life of 6 hours, and then becomes radioactive *Technetium-99 with a much longer half life of 2.13×105 years, which eventually becomes stable Ruthenium-99.
- neucleon – A single neutron cell with 1, 2, or 3 spinning cores below a charge shield. See Diagram The Three Neucleons.
- neucleon plasm number – equal to the neucleon number
- neucleon plasm – The invariant 0.000833u of Type II spinrise matter substance contained within each neucleon cell.
- neucleon plasm volume – The volume of each plasm particle within a nuclide. Plasm changes in volume with nuclide density.
- neucleon membrane – The invariant 0.000544u of Type I spinrise matter membrane surrounding a neucleon.
- neucleon membrane number – equal to the cluster number C.
- neucleon number – C, the total number of neucleon cells packed below a charge shield. The maximum stable neucleon number found in nature is 126 with Lead-208 and Bismuth-209. This number is also the cluster number C.
- neucleon synthesis – The manufacture of deuterons, helions, and neutrons.
- neutron – The neutron (n) is the primal 3 part matter cell, the proto object of nature, and the neutral a-state of matter. The neutron is the quantum whole of nature, and the atomic mass unit in Neu Theory. See Diagram The Quantum Whole. The neutron is stable below the charge shield of a neucleon cluster, however, is unstable as a free cell without a charge shield, and will spin-off its membrane becoming a kinetic proton/electron charge couple, and a quanta of space, the zomon pulse. See (Figure 3.1 – The Little Bang Transformation).
- neutron capture – The addition of a neutron cell below the nuclide charge shield increasing its cluster number by one. The charge number (Z) is unchanged. See Diagram Neutron Capture.
- neutron emission – The ejection of a neutron cell from a nuclide reducing its cluster number by one. For example; the synthesis of 5 neucleon cell *carbon-11, from 6 neucleon cell boron-11, by the capture of a proton, makes a helion within the nuclide with the emission of a neutron. See (Figure 4.16 – Positron Emission).
- neutron number – Is equal to the number of neutron neucleons within a neucleon cluster. Each neutron neucleon adds zero charge to the nuclide as its mass number “A” increases by one, and the atomic number “Z” remains the same.
- nuclide membrane – The portion of the neucleon membrane S3 surface(s) that touch the charge shells.
- nuclide shape – The nuclide (neucleon cluster) shape, is a deformable spheroid made of individual deformable neucleon cells.
- nuclide recoil – The reaction motion of the nuclide, from the de-linkage of a portion of captive proton matter into energy. The rise energy becomes recoil momentum, and the spin energy is emitted as a gamma ray photon in the opposite direction.
- positron – The positron (e+), is a spinning ball of spinrise matter equal in mass to the electron, that is manufactured from the equal reduction in mass of all captive protons, and ejected from the nuclide carrying one positive charge shell. The positron is not anti-matter, just ordinary Type I matter, but it is nature’s “electron killer.”
- positron emission – The radioactive emission of a positron made during the synthesis of a neutron neucleon within a nuclide. See (Figure 4.16 – Positron Emission).
- proton – The proton (p+), is the detached spinning S1 neutron core covered w/ a positive electric charge layer, that remains after membrane spin-off with the little bang. The proton is a quantum part of the b-state of matter.
- proton capture – The capture of a proton by a deuteron in a nuclide making a helion. The proton charge shell is detached and “fused”, meaning it has been added as another charge shell layer, over the existing neucleon cluster charge shield. Proton capture increases both the mass number “A” and the nuclear charge number “Z” by 1. The neucleon number “C” remains the same, as 1 deuteron has become 1 helion. See (Figure 4.6 – The Helion – He3).
- spin/magnetic balance – The net residual internal spin/magnetic state within a nuclide expressed as a positive or negative whole number, depending on the net spin direction. The entire nuclide has a spin/magnetic axis, a composite of the individual neucleon spin numbers. but is not considered to rotate as a body. The neutron and helion spin value is always one (1). The deuteron spin value is always two (2). It is predicted that neucleons can only add, up or down, with these values. It should be noted that in the Neu Theory model, spin is always a whole quantity, unlike current science that considers spin to be a one half quantity, e.g., the 1/2 spin of the proton in current science is 1 spin in this model. Each core contributes one unit spin.
- spontaneous neutron transformation – The spontaneous spin-off and inversion by the neutron membrane into a free electron, a free or neucleon proton, and the de-linkage of hollow plasm matter into environmental energy. The little bang or beta decay. See (Figure 3.1 – The Little Bang Transformation).
